
SL Paper 1
The enthalpy of combustion of ethanol is determined by heating a known mass of tap water in a glass beaker with a flame of burning ethanol.
Which will lead to the greatest error in the final result?
A. Assuming the density of tap water is 1.0 g cm−3
B. Assuming all the energy from the combustion will heat the water
C. Assuming the specific heat capacity of the tap water is 4.18 J g−1 K−1
D. Assuming the specific heat capacity of the beaker is negligible
Markscheme
B
Examiners report
What is the enthalpy of combustion of butane in kJ mol−1?
2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(l)
\[\begin{array}{*{20}{l}} {{\text{C(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}}}&{\Delta H = x{\text{ kJ}}} \\ {{{\text{H}}_2}{\text{(g)}} + \frac{{\text{1}}}{2}{{\text{O}}_2}{\text{(g)}} \to {{\text{H}}_2}{\text{O(l)}}}&{\Delta H = y{\text{ kJ}}} \\ {4{\text{C(s)}} + {\text{5}}{{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_4}{{\text{H}}_{{\text{10}}}}{\text{(g)}}}&{\Delta H = z{\text{ kJ}}} \end{array}\]
A. 4x + 5y − z
B. 4x + 5y + z
C. 8x + 10y − 2z
D. 8x + 5y + 2z
Markscheme
A
Examiners report
A 5.00 g sample of a substance was heated from 25.0 °C to 35.0 °C using \(2.00 \times {10^2}{\text{ J}}\) of energy. What is the specific heat capacity of the substance in \({\text{J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\)?
A. \(4.00 \times {10^{ - 3}}\)
B. \(2.50 \times {10^{ - 1}}\)
C. 2.00
D. 4.00
Markscheme
D
Examiners report
Which statement is correct for this reaction?
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g) ΔH = −26.6 kJ
A. 13.3 kJ are released for every mole of Fe produced.
B. 26.6 kJ are absorbed for every mole of Fe produced.
C. 53.2 kJ are released for every mole of Fe produced.
D. 26.6 kJ are released for every mole of Fe produced.
Markscheme
A
Examiners report
The enthalpy changes for two reactions are given.
Br2 (l) + F2 (g) → 2BrF (g) ΔH = x kJ
Br2 (l) + 3F2 (g) → 2BrF3 (g) ΔH = y kJ
What is the enthalpy change for the following reaction?
BrF (g) + F2 (g) → BrF3 (g)
A. x – y
B. –x + y
C. \(\frac{1}{2}\)(–x + y)
D. \(\frac{1}{2}\)(x – y)
Markscheme
C
Examiners report
A student measured the temperature of a reaction mixture over time using a temperature probe. By considering the graph, which of the following deductions can be made?
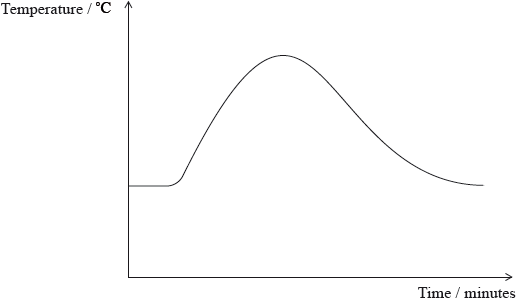
I. The reaction is exothermic.
II. The products are more stable than the reactants.
III. The reactant bonds are stronger than the product bonds.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
There were two G2 comments on this question, both suggesting that the graph given was confusing to candidates. In this question candidates had to use a combination of ideas to ascertain that the correct answer is A, namely I. and II. From the graph shown, candidates need to realise that the reaction is exothermic, and therefore from this information, the products are more stable than the reactants. 55% of candidates got the correct answer.
What is the enthalpy change, in kJ, of the following reaction?
3H2 (g) + N2 (g) \( \rightleftharpoons \) 2NH3 (g)
A. (6 × 391) − [(3 × 436) + 945]
B. (3 × 391) − (436 + 945)
C. −[(3 × 436) + 945] + (3 × 391)
D. −(6 × 391) + [(3 × 436) + 945]
Markscheme
D
Examiners report
Which process is endothermic?
A. \({\text{C}}{{\text{H}}_4}{\text{(g)}} + {\text{2}}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\)
B. \({\text{HCl(aq)}} + {\text{NaOH(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_2}{\text{O(l)}}\)
C. \({\text{CaC}}{{\text{O}}_3}{\text{(s)}} \to {\text{CaO(s)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\)
D. \({{\text{H}}_2}{\text{O(g)}} \to {{\text{H}}_2}{\text{O(l)}}\)
Markscheme
C
Examiners report
Why is the value of the enthalpy change of this reaction calculated from bond enthalpy data less accurate than that calculated from standard enthalpies of formation?
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(g)
A. All the reactants and products are gases.
B. Bond enthalpy data are average values for many compounds.
C. Elements do not have standard enthalpy of formation.
D. Standard enthalpies of formation are per mole.
Markscheme
B
Examiners report
5.35g of solid ammonium chloride, NH4Cl(s), was added to water to form 25.0g of solution. The maximum decrease in temperature was 14 K. What is the enthalpy change, in kJmol-1, for this reaction? (Molar mass of NH4Cl = 53.5gmol-1; the specific heat capacity of the solution is 4.18 Jg-1K-1)
A. \(\Delta H = + \frac{{25.0 \times 4.18 \times \left( {14 + 273} \right)}}{{0.1 \times 1000}}\)
B. \(\Delta H = - \frac{{25.0 \times 4.18 \times 14}}{{0.1 \times 1000}}\)
C. \(\Delta H = + \frac{{25.0 \times 4.18 \times 14}}{{0.1 \times 1000}}\)
D. \(\Delta H = + \frac{{25.0 \times 4.18 \times 14}}{{1000}}\)
Markscheme
C
Examiners report
A simple calorimeter was set up to determine the enthalpy change occurring when one mole of ethanol is combusted. The experimental value was found to be \( - 867{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). The Data Booklet value is \( - 1367{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) (at 298 K and \(1.01 \times {10^5}{\text{ Pa}}\)).
During the experiment some black soot formed.
Which statements are correct?
I. The percentage error for the experiment can be calculated as follows:
\[(1367 - 867) \times 100\% \]
II. The difference between the two values may be due to heat loss to the surroundings.
III. The black soot suggests that incomplete combustion occurred.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
Which describes the reaction shown in the potential energy profile?
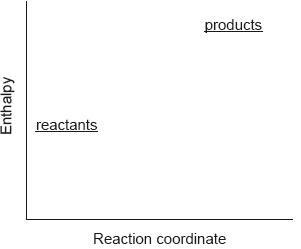
A. The reaction is endothermic and the products have greater enthalpy than the reactants.
B. The reaction is endothermic and the reactants have greater enthalpy than the products.
C. The reaction is exothermic and the products have greater enthalpy than the reactants.
D. The reaction is exothermic and the reactants have greater enthalpy than the products.
Markscheme
A
Examiners report
Which statement is correct?
A. In an exothermic reaction, the products have more energy than the reactants.
B. In an exothermic reversible reaction, the activation energy of the forward reaction is greater than that of the reverse reaction.
C. In an endothermic reaction, the products are more stable than the reactants.
D. In an endothermic reversible reaction, the activation energy of the forward reaction is greater than that of the reverse reaction.
Markscheme
D
Examiners report
Consider the following reactions.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{u}}_2}{\text{O(s)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{2CuO(s)}}}&{\Delta {H^\Theta } = - 144{\text{ kJ}}} \\ {{\text{C}}{{\text{u}}_2}{\text{O(s)}} \to {\text{Cu(s)}} + {\text{CuO(s)}}}&{\Delta {H^\Theta } = + 11{\text{ kJ}}} \end{array}\]
What is the value of \(\Delta {H^\Theta }\), in kJ, for this reaction?
\[{\text{Cu(s)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{CuO(s)}}\]
A. \( - 144 + 11\)
B. \( + 144 - 11\)
C. \( - 144 - 11\)
D. \( + 144 + 11\)
Markscheme
C
Examiners report
One respondent stated that there was too much mathematics required to answer this question. However, candidates simply had to use Hess’s law and were not required to determine the numerical value of the final answer. In fact, the question was the eight easiest question on the paper and 76.61% of candidates got the correct answer C.
Consider the following reactions.
\[\begin{array}{*{20}{l}} {{{\text{N}}_2}({\text{g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2NO(g)}}}&{\Delta {H^\Theta } = + 180{\text{ kJ}}} \\ {2{\text{N}}{{\text{O}}_2}({\text{g)}} \to {\text{2NO(g)}} + {{\text{O}}_2}{\text{(g)}}}&{\Delta {H^\Theta } = + 112{\text{ kJ}}} \end{array}\]
What is the \({\Delta {H^\Theta }}\) value, in kJ, for the following reaction?
\[{{\text{N}}_2}({\text{g)}} + {\text{2}}{{\text{O}}_2}{\text{(g)}} \to {\text{2N}}{{\text{O}}_2}{\text{(g)}}\]
A. \( - 1 \times ( + 180) + - 1 \times ( + 112)\)
B. \( - 1 \times ( + 180) + 1 \times ( + 112)\)
C. \(1 \times ( + 180) + - 1 \times ( + 112)\)
D. \(1 \times ( + 180) + 1 \times ( + 112)\)
Markscheme
C
Examiners report
Two respondents stated that there was too much mathematics required to answer this question. However, candidates simply had to use Hess‟s law and were not required to determine the numerical value of the final answer. In fact, the question was the second easiest question on the paper and 82.41% of candidates got the correct answer C.
Which combination is correct for the exothermic reaction that occurs between zinc and copper sulfate solution.
Markscheme
C
Examiners report
Some water is heated using the heat produced by the combustion of magnesium metal. Which values are needed to calculate the enthalpy change of reaction?
I. The mass of magnesium
II. The mass of the water
III. The change in temperature of the water
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
There was some ambiguity in the wording of the question as to whether the enthalpy change required was that associated with the burning of the particular sample of magnesium, or the molar enthalpy change. For that reason it was decided to accept both response C and response D as correct.
What can be deduced from this reaction profile?
A. The reactants are less stable than the products and the reaction is exothermic.
B. The reactants are less stable than the products and the reaction is endothermic.
C. The reactants are more stable than the products and the reaction is exothermic.
D. The reactants are more stable than the products and the reaction is endothermic.
Markscheme
A
Examiners report
Identical pieces of magnesium are added to two beakers, A and B, containing hydrochloric acid. Both acids have the same initial temperature but their volumes and concentrations differ.
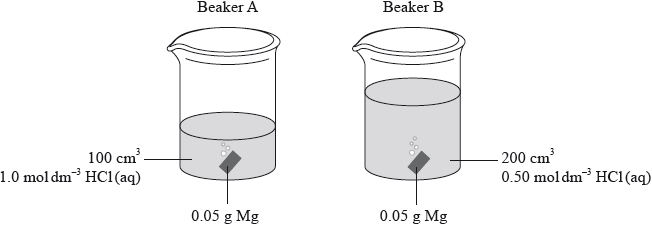
Which statement is correct?
A. The maximum temperature in A will be higher than in B.
B. The maximum temperature in A and B will be equal.
C. It is not possible to predict whether A or B will have the higher maximum temperature.
D. The temperature in A and B will increase at the same rate.
Markscheme
A
Examiners report
One G2 comment stated although this was a good question, it would be challenging for many SL candidates. In fact, although this was the fifth hardest question on the entire paper, 43% of candidates still managed to get the question correct.
Hydrazine reacts with oxygen.
N2H4(l) + O2(g) → N2(g) + 2H2O(l) ΔHθ = -623 kJ
What is the standard enthalpy of formation of N2H4(l) in kJ? The standard enthalpy of formation of H2O(l) is -286 kJ.
A. -623 - 286
B. -623 + 572
C. -572 + 623
D. -286 + 623
Markscheme
C
Examiners report
Two 100 cm3 aqueous solutions, one containing 0.010 mol NaOH and the other 0.010 mol HCl, are at the same temperature.
When the two solutions are mixed the temperature rises by y °C.
Assume the density of the final solution is 1.00 g cm−3.
Specific heat capacity of water = 4.18 J g−1 K−1
What is the enthalpy change of neutralization in kJ mol−1?
A. \(\frac{{200 \times 4.18 \times y}}{{1000 \times 0.020}}\)
B. \(\frac{{200 \times 4.18 \times y}}{{1000 \times 0.010}}\)
C. \(\frac{{100 \times 4.18 \times y}}{{1000 \times 0.010}}\)
D. \(\frac{{200 \times 4.18 \times (y + 273)}}{{1000 \times 0.010}}\)
Markscheme
B
Examiners report
Which processes are exothermic?
I. Ice melting
II. Neutralization
III. Combustion
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
What can be deduced from the facts that ozone absorbs UV radiation in the region of 340 nm and molecular oxygen in the region of 242 nm?
A. The bond between atoms in molecular oxygen is a double bond.
B. The bonds in ozone are delocalized.
C. The bonds between atoms in ozone are stronger than those in molecular oxygen.
D. The bonds between atoms in molecular oxygen need more energy to break.
Markscheme
D
Examiners report
The reaction between methane and oxygen is exothermic.
\({\text{C}}{{\text{H}}_4}({\text{g)}} + {\text{2}}{{\text{O}}_2}({\text{g)}} \to {\text{C}}{{\text{O}}_2}({\text{g)}} + {\text{2}}{{\text{H}}_2}{\text{O}}({\text{g)}}\)
Which statement is correct?
A. The total bond enthalpies of the reactants are less than the total bond enthalpies of the products.
B. The total bond enthalpies of the reactants are greater than the total bond enthalpies of the products.
C. The total energy released during bond formation is less than the total energy absorbed during bond breaking.
D. The activation energy is the difference between the total bond enthalpies of the products and the total bond enthalpies of the reactants.
Markscheme
A
Examiners report
Which statement about bonding is correct?
A. Bond breaking is endothermic and requires energy.
B. Bond breaking is endothermic and releases energy.
C. Bond making is exothermic and requires energy.
D. Bond making is endothermic and releases energy.
Markscheme
A
Examiners report
The specific heat of iron is \({\text{0.450 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\). What is the energy, in J, needed to increase the temperature of 50.0 g of iron by 20.0 K?
A. 9.00
B. 22.5
C. 45.0
D. 450
Markscheme
D
Examiners report
What is the value of \(\Delta H\) for the exothermic reaction represented by the diagram below?
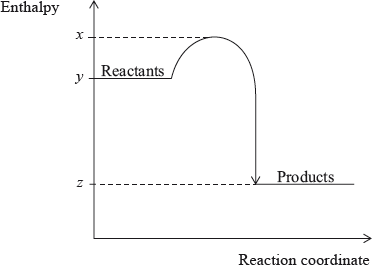
A. \(y - z\)
B. \(z - y\)
C. \(x - z\)
D. \(z - x\)
Markscheme
B
Examiners report
In a reaction that occurs in 50 g of aqueous solution, the temperature of the reaction mixture increases by 20 °C. If 0.10 mol of the limiting reagent is consumed, what is the enthalpy change (in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)) for the reaction? Assume the specific heat capacity of the solution \( = 4.2{\rm{k}}{{\rm{J}}^{ - 1}}{{\rm{K}}^{ - 1}}\).
A. \( - 0.10 \times 50 \times 4.2 \times 20\)
B. \( - 0.10 \times 0.050 \times 4.2 \times 20\)
C. \(\frac{{ - 50 \times 4{\text{.}}2 \times 20}}{{0{\text{.}}10}}\)
D. \(\frac{{ - 0{\text{.}}050 \times 4{\text{.}}2 \times 20}}{{0{\text{.}}10}}\)
Markscheme
D
Examiners report
There was a mistake in the units for heat capacity in this question (\({\text{kJ}}\,{\text{k}}{{\text{g}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\) rather than \({\text{kJ}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\)), but this did not appear to put students off as the Difficulty Index and Discrimination Index were both of the order of magnitude that was anticipated.
What is the temperature rise when 2100 J of energy is supplied to 100 g of water? (Specific heat capacity of water \( = 4.2{\text{ J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\).)
A. 5 °C
B. 278 K
C. 0.2 °C
D. 20 °C
Markscheme
A
Examiners report
This question was designed to test an understanding of difference in temperature. Although 68% gave the correct answer, nearly 20% added 273 (answer B).
Which statement is correct given the enthalpy level diagram below?
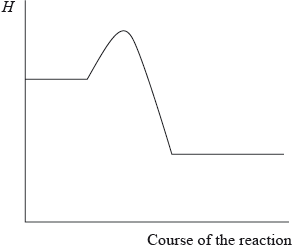
A. The reaction is endothermic and the products are more thermodynamically stable than the reactants.
B. The reaction is exothermic and the products are more thermodynamically stable than the reactants.
C. The reaction is endothermic and the reactants are more thermodynamically stable than the products.
D. The reaction is exothermic and the reactants are more thermodynamically stable than the products.
Markscheme
B
Examiners report
Which of the following reactions are exothermic?
I. \({{\text{C}}{{\text{H}}_{\text{4}}} + {\text{2}}{{\text{O}}_{\text{2}}} \to {\text{C}}{{\text{O}}_{\text{2}}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O}}}\)
II. \({{\text{NaOH}} + {\text{HCl}} \to {\text{NaCl}} + {{\text{H}}_2}{\text{O}}}\)
III. \({{\text{B}}{{\text{r}}_2} \to 2{\text{Br}}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
The specific heat capacities of two substances are given in the table below.

Which statement is correct?
A. More heat is needed to increase the temperature of 50 g of water by 50 °C than 50 g of ethanol by 50 °C.
B. If the same heat is supplied to equal masses of ethanol and water, the temperature of the water increases more.
C. If equal masses of water at 20 °C and ethanol at 50 °C are mixed, the final temperature is 35 °C .
D. If equal masses of water and ethanol at 50 °C cool down to room temperature, ethanol liberates more heat.
The enthalpy changes of three reactions are given below.
\({\text{2HCOOH(l)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\) \(\Delta H = a\)
\({{\text{C}}_2}{{\text{H}}_5}{\text{OH(l)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}\) \(\Delta H = b\)
\({\text{2HCOO}}{{\text{C}}_2}{{\text{H}}_5}{\text{(l)}} + {\text{7}}{{\text{O}}_2}{\text{(g)}} \to {\text{6C}}{{\text{O}}_2}{\text{(g)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}}\) \(\Delta H = c\)
What is the enthalpy change for the following reaction?
\[{\text{HCOOH(l)}} + {{\text{C}}_2}{{\text{H}}_5}{\text{OH(l)}} \to {\text{HCOO}}{{\text{C}}_2}{{\text{H}}_5}{\text{(l)}} + {{\text{H}}_2}{\text{O(l)}}\]
A. \(a + b + c\)
B. \(a + 2b - c\)
C. \(\frac{1}{2}a + b + \frac{1}{2}c\)
D. \(\frac{1}{2}a + b - \frac{1}{2}c\)
Markscheme
A
D
Examiners report
What is the enthalpy change of combustion of urea, (NH2)2CO, in kJ mol−1?
2(NH2)2CO(s) + 3O2(g) → 2CO2(g) + 2N2(g) + 4H2O(l)
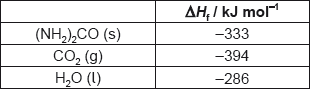
A. 2 × (−333) −2 × (−394) −4 × (−286)
B. \(\frac{1}{2}\)[2 × (−394) + 4 × (−286) −2 × (−333)]
C. 2 × (−394) + 4 × (−286) −2 × (−333)
D. \(\frac{1}{2}\)[2 × (−333) −2 × (−394) −4 × (−286)]
Markscheme
B
Examiners report
Which is correct about energy changes during bond breaking and bond formation?
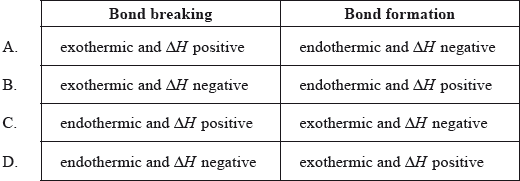
Markscheme
C
Examiners report
In which order does the oxygen–oxygen bond enthalpy increase?
A. H2O2 < O2 < O3
B. H2O2 < O3 < O2
C. O2 < O3 < H2O2
D. O3 < H2O2 < O2
Markscheme
B
Examiners report
Which change of state is exothermic?
A. CO2(s) → CO2(g)
B. H2O(l) → H2O(g)
C. NH3(g) → NH3(l)
D. Fe(s) → Fe(l)
Markscheme
C
Examiners report
Which process represents the C–Cl bond enthalpy in tetrachloromethane?
A. \({\text{CC}}{{\text{l}}_{\text{4}}}{\text{(g)}} \to {\text{C(g)}} + {\text{4Cl(g)}}\)
B. \({\text{CC}}{{\text{l}}_4}({\text{g)}} \to {\text{CC}}{{\text{l}}_3}({\text{g)}} + {\text{Cl(g)}}\)
C. \({\text{CC}}{{\text{l}}_4}({\text{l)}} \to {\text{C(g)}} + 4{\text{Cl(g)}}\)
D. \({\text{CC}}{{\text{l}}_4}({\text{l)}} \to {\text{C(s)}} + 2{\text{C}}{{\text{l}}_2}({\text{g)}}\)
Markscheme
B
Examiners report
The difficulty index for this question was 35% with both answers A and D providing very attractive discriminators. Though the bond enthalpy can be determined in terms of the change in response A, candidates should have realised that the magnitude of the associated enthalpy change would be approximately four times greater than that required to break a single C-Cl bond.
When 25.0cm3 0.100moldm−3 NaOH(aq) is mixed with 25.0cm3 0.100moldm−3 HCl(aq) at the same temperature, a temperature rise, ∆T, is recorded. What is the expression, in kJ mol−1, for the enthalpy of neutralisation? (Assume the density of the mixture = 1.00 g cm−3 and its specific heat capacity=4.18kJkg−1K−1 =4.18Jg−1K−1)
A. \( - \frac{{25.0 \times 4.18 \times \Delta T}}{{50.0 \times 0.100}}\)
B. \( - \frac{{25.0 \times 4.18 \times \Delta T}}{{25.0 \times 0.100}}\)
C. \( - \frac{{50.0 \times 4.18 \times \Delta T}}{{50.0 \times 0.100}}\)
D. \( - \frac{{50.0 \times 4.18 \times \Delta T}}{{25.0 \times 0.100}}\)
Markscheme
D
Examiners report
Which expression gives the mass, in g, of ethanol required to produce 683.5 kJ of heat upon complete combustion?
(Mr for ethanol = 46.0, \(\Delta H_c^\theta = - 1367{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\))
A. \(\frac{{683.5}}{{1367 \times 46.0}}\)
B. \(\frac{{1367}}{{683.5 \times 46.0}}\)
C. \(\frac{{683.5 \times 46.0}}{{1367}}\)
D. \(\frac{{1367 \times 46.0}}{{683.5}}\)
Markscheme
C
Examiners report
Using the equations below:
\[\begin{array}{*{20}{l}} {{\text{C(s)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\Theta } = - 390{\text{ kJ}}} \\ {{{\text{H}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 286{\text{ kJ}}} \\ {{\text{C}}{{\text{H}}_4}{\text{(g)}} + {\text{2}}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 890{\text{ kJ}}} \end{array}\]
what is \({\Delta {H^\Theta }}\), in kJ, for the following reaction?
\[{\text{ C(s)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \to {\text{C}}{{\text{H}}_4}{\text{(g)}}\]
A. –214
B. –72
C. +72
D. +214
Markscheme
B
Examiners report
The examination normally uses kJ when an equation is given as here; if it were to quote a value, say, for the heat of formation of a compound, then this would be given in kJ\(\,\)mol–1.
Consider the following enthalpy of combustion data.
\[\begin{array}{*{20}{l}} {{\text{C(s)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\Theta } = - x{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{{\text{H}}_2}{\text{(g) + }}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - y{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{{\text{C}}_2}{{\text{H}}_6}{\text{(g)}} + {\text{3}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g) + 3}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - z{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
What is the enthalpy of formation of ethane in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)?
\[{\text{2C(s)}} + {\text{3}}{{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_6}{\text{(g)}}\]
A. \(\left[ {( - x) + ( - y)} \right] - ( - z)\)
B. \(( - z) - \left[ {( - x) + ( - y)} \right]\)
C. \(\left[ {( - 2x) + ( - 3y)} \right] - ( - z)\)
D. \(( - z) - \left[ {( - 2x) + ( - 3y)} \right]\)
Markscheme
C
Examiners report
There were three G2 comments on this question on Hess’s law, all of which stated that giving x, y and z variables instead of numeric data was confusing. However, candidates do not have the use of a calculator in P1 and hence it is common practice to use algebraic notation for this purpose. This notation has been used previously in P1 (though not always). In addition, this is a very common question and in fact, candidates had no problem whatsoever answering this question, with 80% getting the correct answer, C. The question was the third easiest question on the paper.
B. \(\frac{1}{4}\) SiH4 (g) → \(\frac{1}{4}\) Si(g) + H(g)
C. SiH4(g) → SiH3(g) + \(\frac{1}{2}\) H2(g)
D. SiH4 (g) → Si(g) + 4H(g)
Markscheme
B
Examiners report
In which reaction do the reactants have a lower potential energy than the products?
A. CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
B. HBr(g) → H(g) + Br(g)
C. Na+(g) + Cl-(g) → NaCl(s)
D. NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Markscheme
B
Examiners report
What is the energy, in kJ, released when 1.00 mol of carbon monoxide is burned according to the following equation?
\[\begin{array}{*{20}{l}} {{\text{2CO(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2C}}{{\text{O}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\Theta } = - 564{\text{ kJ}}} \end{array}\]
A. 141
B. 282
C. 564
D. 1128
Markscheme
B
Examiners report
Which statements are correct for an exothermic reaction?
I. The products are more stable than the reactants.
II. The enthalpy change, \(\Delta H\), is negative.
III. The temperature of the surroundings increases.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
When \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) HCl is mixed with \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) NaOH, the temperature of the resulting solution increases by 5.0 °C. What will be the temperature change, in °C, when \({\text{50 c}}{{\text{m}}^{\text{3}}}\) of these two solutions are mixed?
A. 2.5
B. 5.0
C. 10
D. 20
Markscheme
B
Examiners report
There were three G2 comments on this question. Some suggested that it would be better if more consistent use of significant figures would be used, which is noted and one stated that the question was complicated. The question was certainly challenging for candidates and only 36.27% of candidates got the correct answer B.
Consider the following two equations.
\({\text{2Ca(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2CaO(s)}}\) \(\Delta {H^\Theta } = + x{\text{ kJ}}\)
\({\text{Ca(s)}} + {\text{0.5}}{{\text{O}}_2}{\text{(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} \to {\text{CaC}}{{\text{O}}_3}{\text{(s)}}\) \(\Delta {H^\Theta } = + y{\text{ kJ}}\)
What is \(\Delta {H^\Theta }\), in kJ, for the following reaction?
\[{\text{CaO(s)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} \to {\text{CaC}}{{\text{O}}_3}{\text{(s)}}\]
A. \(y - 0.5x\)
B. \(y - x\)
C. \(0.5 - y\)
D. \(x - y\)
Markscheme
A
Examiners report
There was concern about the use of algebraic notation rather than actual numerical data. This has been used since November 2010 so candidates should be familiar with this type of question. (In fact, some G2s in the past have suggested it would be better to use algebraic notation!) In the event it was the sixth easiest question; over 81% of candidates gave the correct answer and 9% gave B.
Which processes have a negative enthalpy change?
I. \(2{\text{C}}{{\text{H}}_3}{\text{OH(l)}} + 3{{\text{O}}_2}({\text{g)}} \to {\text{2C}}{{\text{O}}_2}({\text{g)}} + 4{{\text{H}}_2}{\text{O(l)}}\)
II. \({\text{HCl(aq)}} + {\text{NaOH(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_2}{\text{O(l)}}\)
III. \({{\text{H}}_2}{\text{O(g)}} \to {{\text{H}}_2}{\text{O(l)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
When some solid barium hydroxide and solid ammonium thiosulfate were reacted together, the temperature of the surroundings was observed to decrease from 15 °C to –4 °C. What can be deduced from this observation?
A. The reaction is exothermic and \(\Delta H\) is negative.
B. The reaction is exothermic and \(\Delta H\) is positive.
C. The reaction is endothermic and \(\Delta H\) is negative.
D. The reaction is endothermic and \(\Delta H\) is positive.
Markscheme
D
Examiners report
At 25 °C, \({\text{200 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) nitric acid is added to 5.0 g of magnesium powder. If the experiment is repeated using the same mass of magnesium powder, which conditions will result in the same initial reaction rate?
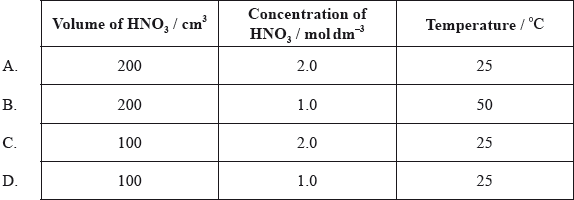
Markscheme
D
Examiners report
When four moles of aluminium and four moles of iron combine with oxygen to form their oxides, the enthalpy changes are –3338 kJ and –1644 kJ respectively.
\({\text{4Al(s)}} + {\text{3}}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(s)}}\) \(\Delta H = - 3338{\text{ kJ}}\)
\({\text{4Fe(s)}} + {\text{3}}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(s)}}\) \(\Delta H = - 1644{\text{ kJ}}\)
What is the enthalpy change, in kJ, for the reduction of one mole of iron(III) oxide by aluminium?
\[{\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(s)}} + {\text{2Al(s)}} \to {\text{2Fe(s)}} + {\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(s)}}\]
A. \( + 1694\)
B. \( + 847\)
C. \( - 847\)
D. \( - 1694\)
Markscheme
C
Examiners report
There was one concern about how students of mathematical studies might fare with this question. The arithmetic is straightforward and, in the event, nearly 64% gave the correct answer.
Which processes are exothermic?
I. \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(g)}}\)
II. \({\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{2Cl(g)}}\)
III. \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH(aq)}} + {\text{NaOH(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COONa(aq)}} + {{\text{H}}_2}{\text{O(l)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Which combination is correct for the standard enthalpy change of neutralization?
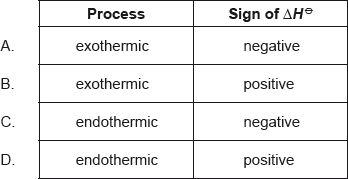
Markscheme
A
Examiners report
The table shows information about temperature increases when an acid and an alkali are mixed.
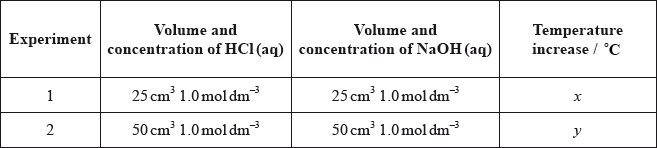
What is the value of \(y\)?
A. \(\frac{1}{2}x\)
B. \(x\)
C. \(2x\)
D. \(4x\)
Markscheme
B
Examiners report
Which processes are exothermic?
I. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH(aq)}} + {\text{NaOH(aq)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{COONa(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\)
II. \({\text{2C(s)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2CO(g)}}\)
III. \({\text{C(s)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
Students found this question also to be difficult with 35.83% correct answers. C was by far the most common answer suggesting that students were aware of combustion being an exothermic process but not neutralization.
Which equation best represents the bond enthalpy of HCl?
A. \({\text{HCl(g)}} \to {{\text{H}}^ + }{\text{(g)}} + {\text{C}}{{\text{l}}^ - }{\text{(g)}}\)
B. \({\text{HCl(g)}} \to {\text{H(g)}} + {\text{Cl(g)}}\)
C. \({\text{HCl(g)}} \to \frac{{\text{1}}}{2}{{\text{H}}_2}({\text{g)}} + \frac{1}{2}{\text{C}}{{\text{l}}_2}({\text{g)}}\)
D. \({\text{2HCl(g)}} \to {{\text{H}}_2}({\text{g)}} + {\text{C}}{{\text{l}}_2}({\text{g)}}\)
Markscheme
B
Examiners report
Which combination is correct about the energy changes during bond breaking and bond formation?
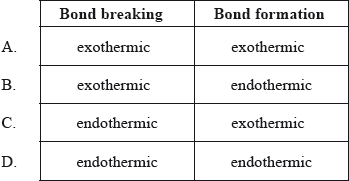
Markscheme
C
Examiners report
The specific heat capacity of aluminium is \({\text{0.900 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\). What is the heat energy change, in J, when 10.0 g of aluminium is heated and its temperature increases from 15.0 °C to 35.0 °C?
A. +180
B. +315
C. +1800
D. +2637
Markscheme
A
Examiners report
The standard enthalpy changes for the combustion of carbon and carbon monoxide are shown below.
\[\begin{array}{*{20}{l}} {{\text{C(s)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}}}&{\Delta H_{\text{c}}^\Theta = - 394{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{CO(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}}}&{\Delta H_{\text{c}}^\Theta = - 283{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
What is the standard enthalpy change, in kJ, for the following reaction?
\[{\text{C(s)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{CO(g)}}\]
A. –677
B. –111
C. +111
D. +677
Markscheme
B
Examiners report
Which is true for a chemical reaction in which the products have a higher enthalpy than the reactants?
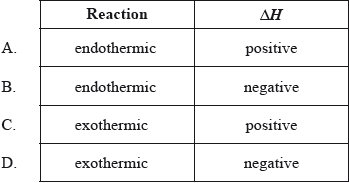
Markscheme
A
Examiners report
Which statement is correct for the enthalpy level diagram shown?
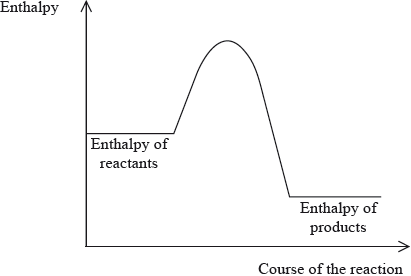
A. The reaction is exothermic and the products are more stable than the reactants.
B. The reaction is exothermic and the sign of the enthalpy change is positive.
C. The reaction is endothermic and the sign of the enthalpy change is negative.
D. The reaction is endothermic and the products are more stable than the reactants.
Markscheme
A
Examiners report
Which statement is correct for the reaction with this enthalpy level diagram?
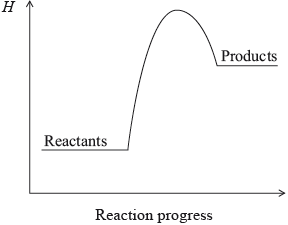
A. Heat energy is released during the reaction and the reactants are more stable than the products.
B. Heat energy is absorbed during the reaction and the reactants are more stable than the products.
C. Heat energy is released during the reaction and the products are more stable than the reactants.
D. Heat energy is absorbed during the reaction and the products are more stable than the reactants.
Markscheme
B
Examiners report
Which equation corresponds to the bond enthalpy of the H–I bond?
A. \({\text{HI(g)}} \to \frac{1}{2}{{\text{H}}_{\text{2}}}{\text{(g)}} + \frac{1}{2}{{\text{I}}_{\text{2}}}{\text{(g)}}\)
B. \({\text{HI(g)}} \to \frac{1}{2}{{\text{H}}_{\text{2}}}{\text{(g)}} + \frac{1}{2}{{\text{I}}_{\text{2}}}{\text{(s)}}\)
C. \({\text{HI(g)}} \to {{\text{H}}^ + }{\text{(g)}} + {{\text{I}}^ - }{\text{(g)}}\)
D. \({\text{HI(g)}} \to {\text{H(g)}} + {\text{I(g)}}\)
Markscheme
D
Examiners report
Students found this question to be the most difficult question with only 10.82% correct answers. This was very surprising considering this was a definition question on bond enthalpy which involves the energy needed to break one mole of the bonds to form separated atoms with reactants and products in the gas state.
Consider the following equations.
\({\text{2Fe(s)}} + {\text{1}}\frac{1}{2}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(s) }}\) \(\Delta {{\text{H}}^\Theta } = x\)
\({\text{CO(g)}} + \frac{1}{2}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}}\) \(\Delta {{\text{H}}^\Theta } = y\)
What is the enthalpy change of the reaction below?
\[{\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(s)}} + {\text{3CO(g)}} \to {\text{3C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{2Fe(s)}}\]
A. \(3y - x\)
B. \(3y + x\)
C. \( - 3y - x\)
D. \( - 3y + x\)
Markscheme
A
Examiners report
This question was answered correctly by nearly 85% of the candidates which was encouraging.
Use the average bond enthalpies below to calculate the enthalpy change, in kJ, for the following reaction.
\[{{\text{H}}_2}{\text{(g)}} + {{\text{I}}_2}{\text{(g)}} \to {\text{2HI(g)}}\]
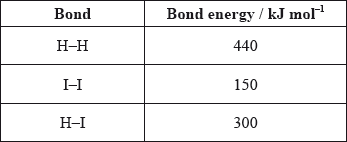
A. +290
B. +10
C. –10
D. –290
Markscheme
C
Examiners report
One G2 comment stated that the question should have asked for the enthalpy change in the reaction in units of \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) instead of kJ. This aspect of units has been discussed in detail in several previous subject reports and teachers are referred to these reports for further reference.
The enthalpy change for the reaction between zinc metal and copper(II) sulfate solution is \(-217{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Which statement about this reaction is correct?
A. The reaction is endothermic and the temperature of the reaction mixture initially rises.
B. The reaction is endothermic and the temperature of the reaction mixture initially drops.
C. The reaction is exothermic and the temperature of the reaction mixture initially rises.
D. The reaction is exothermic and the temperature of the reaction mixture initially drops.
Markscheme
C
Examiners report
Answer D was the most common error.
Consider the equations:
\[\begin{array}{*{20}{l}} {{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(l)}}}&{\Delta {H^\Theta } = + 50.6{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{{\text{N}}_2}{{\text{H}}_4}({\text{l)}} \to {{\text{N}}_2}{{\text{H}}_4}({\text{g)}}}&{\Delta {H^\Theta } = + 44.8{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
What is \({\Delta {H^\Theta }}\), in kJ, for the following reaction?
\[{{\text{N}}_2}({\text{g)}} + 2{{\text{H}}_2}({\text{g)}} \to {{\text{N}}_2}{{\text{H}}_4}({\text{g)}}\]
A. \( - 95.4\)
B. \( - 5.80\)
C. \( + 5.80\)
D. \( + 95.4\)
Markscheme
D
Examiners report
There were two G2 comments on this question, with both saying that the question was too difficult for SL candidates, especially without the use of a calculator. This type of question has been asked on P1 several times before, and this in general was not an issue at all for candidates, with 74.00% of candidates getting the correct answer D. It is true that algebraic variables could have been used, though in this case the calculation involved is relatively simple: \( + 50.6 + ( + 44.8) = + 95.4{\text{ kJ}}\) and is simply the addition of two numbers, since no equation inversion is involved nor is a multiplication factor necessary.
Which expression gives the enthalpy change, ΔH, for the thermal decomposition of calcium carbonate?
A. ΔH = ΔH1 − ΔH2
B. ΔH = 2ΔH1 − ΔH2
C. ΔH = ΔH1 − 2ΔH2
D. ΔH = ΔH1 + ΔH2
Markscheme
A
Examiners report
Which enthalpy changes can be calculated using only bond enthalpy data?
I. \({{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\)
II. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH(l)}} + {\text{3}}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{O(g)}}\)
III. \({\text{C}}{{\text{H}}_{\text{4}}}{\text{(g)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl(g)}} + {\text{HCl(g)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
It should have been clear to candidates that liquid ethanol would require more than bond enthalpy data provided they knew the definition of the latter. 52% gave the correct answer.
B. −788−286−1301
C. +788+286−1301
D. +788+286+1301
Markscheme
A
Examiners report
The C=N bond has a bond length of 130 pm and an average bond enthalpy of 615kJmol-1. Which values would be most likely for the C-N bond?
Markscheme
A